CAR T-Cell Therapy
Reviewed by: HU Medical Review Board | Last reviewed: June 2025 | Last updated: July 2025
Chimeric antigen receptor (CAR) T-cell therapy is a newer treatment for blood cancer. It is a type of immunotherapy that attacks cancer cells directly.1,2
Not all blood cancers can be treated with CAR T-cell therapy at this time, but the list of cancers it treats is rapidly growing. New therapy options are being studied. Currently, approved CAR T-cell therapies are available to treat certain forms of blood cancer. This includes certain forms of acute lymphocytic leukemia (ALL), non-Hodgkin lymphoma (NHL), and multiple myeloma.1,2
The groups of people who can use CAR T-cell therapy are also growing. Some CAR T-cell therapies can be used by both adults and children. In many cases, CAR T-cell therapy is used specifically for cancers that have returned or not responded to other treatments.1,2
This or That
Have you undergone CAR-T cell therapy?
What are T-cells?
T-cells are a type of white blood cell. White blood cells are part of the immune system. Different white blood cells have different roles in preventing infection and illness. T-cells can directly kill cells infected with viruses and cancer cells. They also help B-cells (another type of immune cell) make antibodies. Antibodies are proteins that help fight a variety of infections.1,3
While T-cells can kill cancer cells, cancer cells can sometimes evade immune system detection or decrease the immune response. Many cancer cells have adapted or found ways to stop or slow down normal T-cell function. However, CAR T-cell therapy can help program T-cells to best target cancer cells.1,3
How does CAR T-cell therapy work?
CAR T-cell therapy is a type of immunotherapy. This means it boosts the body’s immune system to better fight cancer. Another term for CAR T-cell therapy is cell-based gene therapy.1-3
Each T-cell has proteins on its surface called antigen receptors. Antigens are proteins on the outside of infectious or cancerous cells. T-cells have different receptors that recognize specific antigens. When a receptor on a T-cell finds its matching antigen, the T-cell is signaled to attack. This helps T-cells kill specific foreign invaders or cancers.1-3
CAR T-cell therapy involves changing T-cell receptors outside the body in a lab. The T-cells are modified and given new, cancer-recognizing receptors. These specific receptors are called chimeric antigen receptors (CARs). The modified T-cells can then be given back to a person so they target and kill cancer cells.1-3
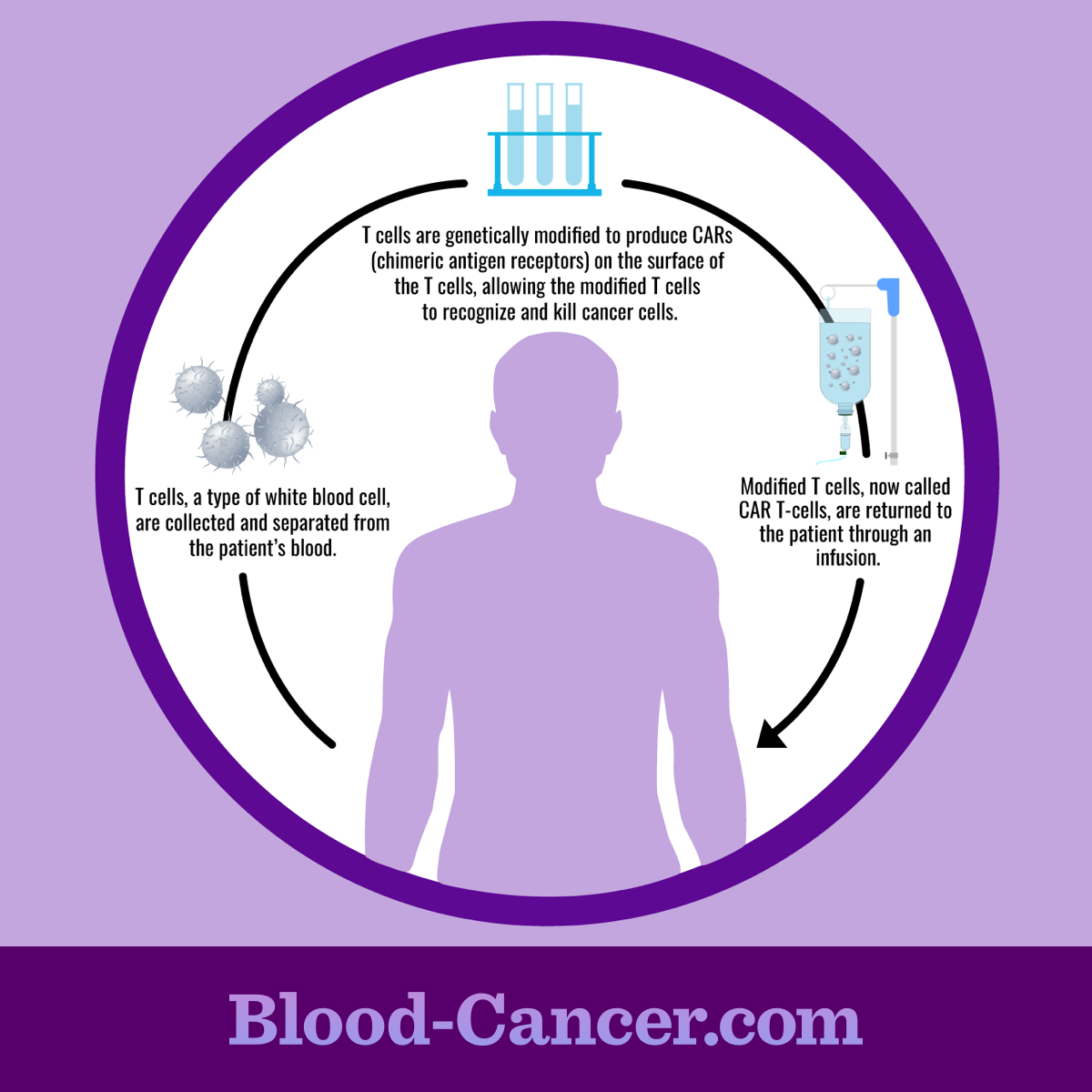
The process
CAR T-cell therapy uses a person’s own immune cells. During the first part of the process, a person’s white blood cells are collected. This is called leukapheresis. It involves removing blood, separating the white blood cells, and then returning the blood back to the body. This is done using 1 or more intravenous (IV) lines. Once the white blood cells are removed from the blood, the T-cells are further separated.1-3
The T-cells are then modified in a lab. They are given the new receptors and allowed to multiply, typically for several weeks. Once enough cells have grown, they are given back to the person they were collected. Before the modified T-cells are given back, a person may first receive chemotherapy. This decreases the number of other white blood cells in the body. This gives the modified T-cells a better chance of working.1-3
Figure 1. CAR T-cell therapy procedure
Examples of CAR T-cell therapy
CAR T-cell therapy is an active area of research. New treatment options are currently in development. Examples of CAR T-cell therapies currently available include:1,3
- Abecma® (idecabtagene vicleucel)
- Aucatzyl® (obecabtagene autoleucel)
- Breyanzi® (lisocabtagene maraleucel)
- Carvykti™ (ciltacabtagene autoleucel)
- Kymriah™ (tisagenlecleucel)
- Tecartus™ (brexucabtagene autoleucel)
- Yescarta® (axicabtagene ciloleucel)
Each CAR T-cell therapy is used for a specific type of cancer and group of people. Talk with your doctor about your cancer and the CAR T-cell options available to see if one is right for you. There are also several clinical trials for new CAR T-cell therapies. If you are interested in taking part in a clinical trial, talk with your doctor.
What are the possible side effects?
Side effects can vary depending on the specific drug you are taking.
CAR T-cell therapy may cause cytokine release syndrome (CRS). Cytokines are proteins involved in the immune response. They help boost the immune system as the new T-cells find and kill cancer cells. However, this can result in harmful side effects. Signs of CRS include:1-3
- Fever and chills
- Nausea, vomiting, or diarrhea
- Dizziness
- Headaches
- Fatigue
- Trouble breathing
- Fast heartbeat or other heart problems
- Muscle or joint pain
- Low blood pressure
CAR T-cell therapy may also cause neurological issues, including:1-3
- Changes in consciousness
- Trouble understanding or producing speech
- Confusion or hallucinations
- Seizures
- Shakiness or tremors
- Trouble balancing
Leukapheresis may decrease calcium levels. Signs of low calcium include muscle spasms, twitching, or numbness. Tell your doctor if you have any of these symptoms.1-3
Some people have an allergic reaction to CAR T-cell therapy. It also may increase your risk of infection or bleeding. Your doctor will monitor you for these side effects.1-3
The following CAR T-cell therapies have boxed warnings, the strictest warning from the US Food and Drug Administration (FDA):4-10
- Abecma
- Aucatzyl
- Breyanzi
- Carvykti
- Kymriah
- Tecartus
- Yescarta
They have these warnings because they may cause severe side effects, including:4-10
- A potentially fatal or life-threatening reaction called cytokine release syndrome (CRS). CRS is a condition that may develop when your immune system overreacts and releases too many cytokines.
- Potentially fatal or life-threatening neurologic toxicities.
- An increased risk of developing a new, unrelated blood cancer (second cancer).
These are not all the possible side effects of CAR T-cell therapy. Some CAR T-cell therapies may also have other boxed warnings in addition to those listed above. Talk to your doctor about what to expect when being treated with CAR T-cell therapy, specifically the specific form you are receiving. You should also call your doctor if you have any changes that concern you during treatment with CAR T-cell therapy.
Other things to know
CAR T-cell therapy is not available everywhere. Only certain cancer centers with experience in CAR T-cell therapy and/or access to clinical trials may be able to complete the process. Many people undergoing CAR T-cell therapy have to travel to a treatment location.2
CAR T-cell therapy can take several weeks to complete. You may need to stay near the hospital where your treatment was given for several weeks afterward. This is generally to monitor for serious side effects.2
Before beginning treatment for blood cancer, tell your doctor about all your health conditions and any other drugs, vitamins, or supplements you are taking. This includes over-the-counter drugs.