Sponsored: A Fifth-Generation Farmer’s Journey With DLBCL
By MorphoSys & Incyte

John, a patient with diffuse large B-cell lymphoma (DLBCL) and his wife, Candace
When John, a fifth-generation family farmer, had an unusual feeling on the right side of his face in 2016, he thought it was a normal ache. Wanting to be safe, he and his wife of more than 50 years, Candace, visited the doctor to have his symptoms examined. Tests revealed that John’s ache was a result of something much more serious: a type of blood cancer called diffuse large B-cell lymphoma (or DLBCL).
DLBCL affects approximately 28,000 people per year in the United States and is the most common type of non-Hodgkin lymphoma (NHL), a family of blood cancers. It is a fast-growing but treatable cancer affecting B-lymphocytes, also known as B cells, a type of white blood cell that helps the body fight infections. As they develop, cancerous B cells become larger than normal and multiply uncontrollably.
After working through the diagnosis, John and Candace committed themselves to being positive and tackling this hurdle as a family. As parents of two and grandparents of six living in a tight-knit community in west central Indiana, there were many things that they wanted to do, and letting a cancer diagnosis control their lives was not one of them.
“Once we accepted my DLBCL diagnosis, we didn’t dwell on it,” John says. “We knew that we had to buck up, stay upbeat and keep moving forward. We sought out a care team that would support us in this approach and found one – our doctors were very diligent.”
“From the beginning, we told our friends and family that we were not going to let cancer dictate our lives,” Candace says. “John’s diagnosis was certainly a bump in the road, but we had our lives to live, and now cancer was just a part of it.”
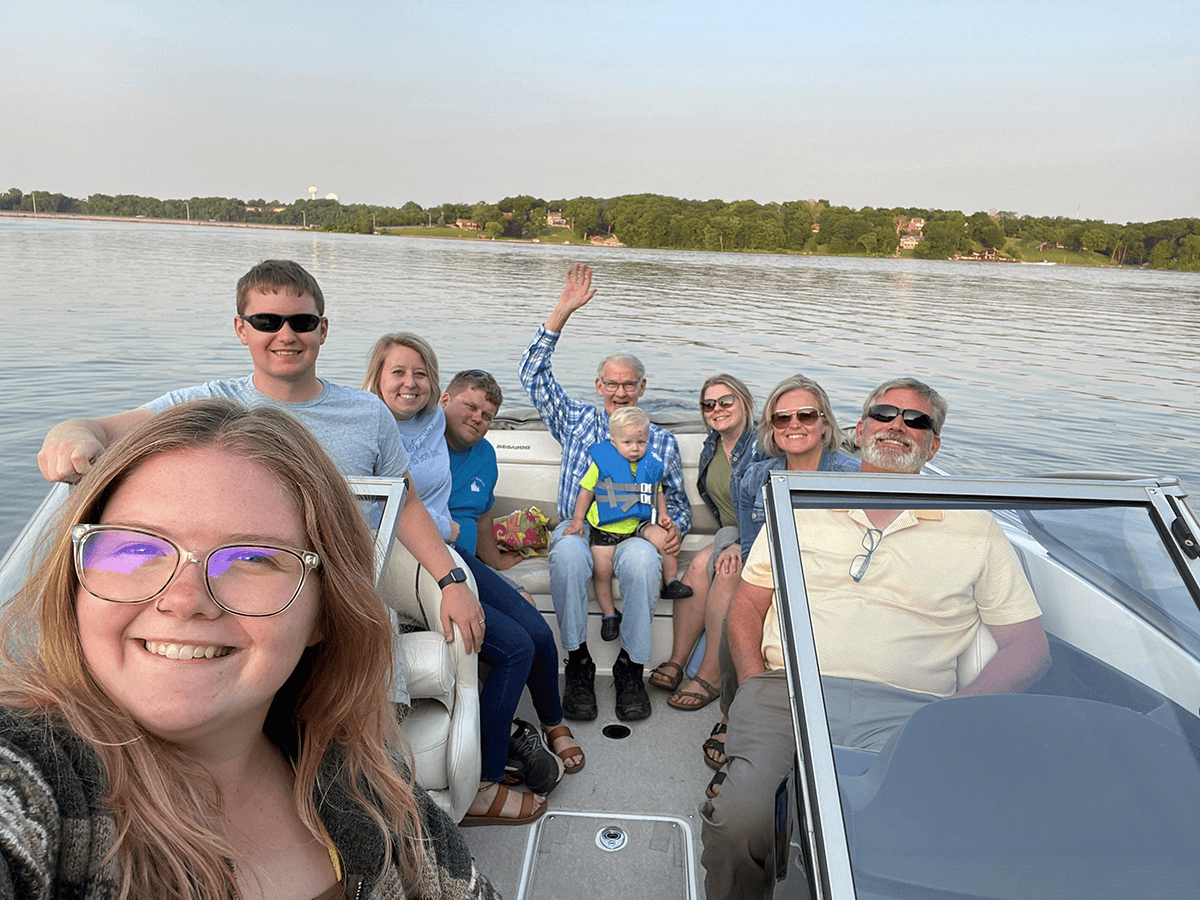
John sharing his passion for boating with his family
John’s care team worked quickly to assess and provide him with the therapy he needed, and after conducting many tests, they performed surgery to remove the cancerous lymph nodes. Following surgery, they initiated treatment with a standard chemoimmunotherapy regimen, and after several rounds, scans revealed he was in remission (a decrease in or disappearance of signs and symptoms of cancer).
However, four years later, John began having a familiar aching sensation – this time on the left side of his face. John and Candace again visited their doctor, who conducted a biopsy that confirmed John’s DLBCL had returned. While this was tough news to receive, John and Candace learned from John's care team that there were still treatment options available and a recurrence of this type of cancer was not uncommon. In fact, up to 50% of patients with DLBCL experience a relapse (cancer returns) following initial treatment or do not respond (cancer becomes refractory) to initial treatment.
John’s care team discussed with him and Candace possible next steps in his treatment, including a stem cell transplant, a procedure in which a patient receives healthy stem cells to replace damaged ones. His care team advised against this because of his pacemaker, so they decided the next option they would try was Monjuvi® (tafasitamab-cxix), a targeted immunotherapy treatment given with another medicine called lenalidomide to treat adults with certain types of DLBCL that have come back or that did not respond to previous treatment and who cannot receive a stem cell transplant. The approval of Monjuvi is based on a type of response rate. There is an ongoing study to confirm the clinical benefit of Monjuvi.
John responded well to Monjuvi, and he and Candace received encouraging news that his cancer was showing improvement. John continues treatment with Monjuvi and is under the care of his physicians. This is John's experience with Monjuvi and reflects results as of the date of this article. Every individual is different and results may vary.
Treatment with Monjuvi does not require hospitalization, and John was able to access the treatment through his local care team, which was his preference as it meant he and Candace wouldn’t have to leave their family or travel long distances.
“For 150 years, our family has been living and farming here,” John says. “We are a proud ‘Hoosier Homestead,’ which is a recognition Indiana gives to various tenures of family farms. My son and grandson have taken over the business now, and we love this area and want to spend as much time here and with our family as possible.”

Four generations: John with his son, grandson and great-grandson
“For John to be able to receive his treatments without traveling many hours has been critical,” Candace states. “It’s made the difference in allowing us more time to do the things we love – we recently helped host 40-60 family members at our annual family reunion on our property.”
Monjuvi may cause serious side effects, including infusion reactions, low blood cell counts and serious infections. The most common side effects of Monjuvi are feeling tired or weak, diarrhea, cough, fever, swelling of lower legs or hands, respiratory tract infection and decreased appetite. These are not all of the possible side effects of Monjuvi. Continue reading to learn more about these and other side effects.
John and Candace’s advice to others is to get checked out if something feels off, stay strong and positive and find a care team that you trust.
“Before being diagnosed, we would not have guessed my ache was related to cancer,” John says. “But we had a feeling and knew it was important to have my symptoms examined by a doctor.”
“It’s so important that you find a care team that is going to work for you,” Candace says. “At our first appointment, we were seen by a nurse practitioner who was determined to find an answer to John’s symptom. He went above and beyond, working with all sorts of different technicians and tests. We consider it a blessing that he and his colleagues were thorough, and we want to send them a heartfelt thank you.”
After three years on Monjuvi, John is on a mission to spend time with Candace and his family in the home they love. Please read the Important Safety Information below to learn more about the side effects of Monjuvi.
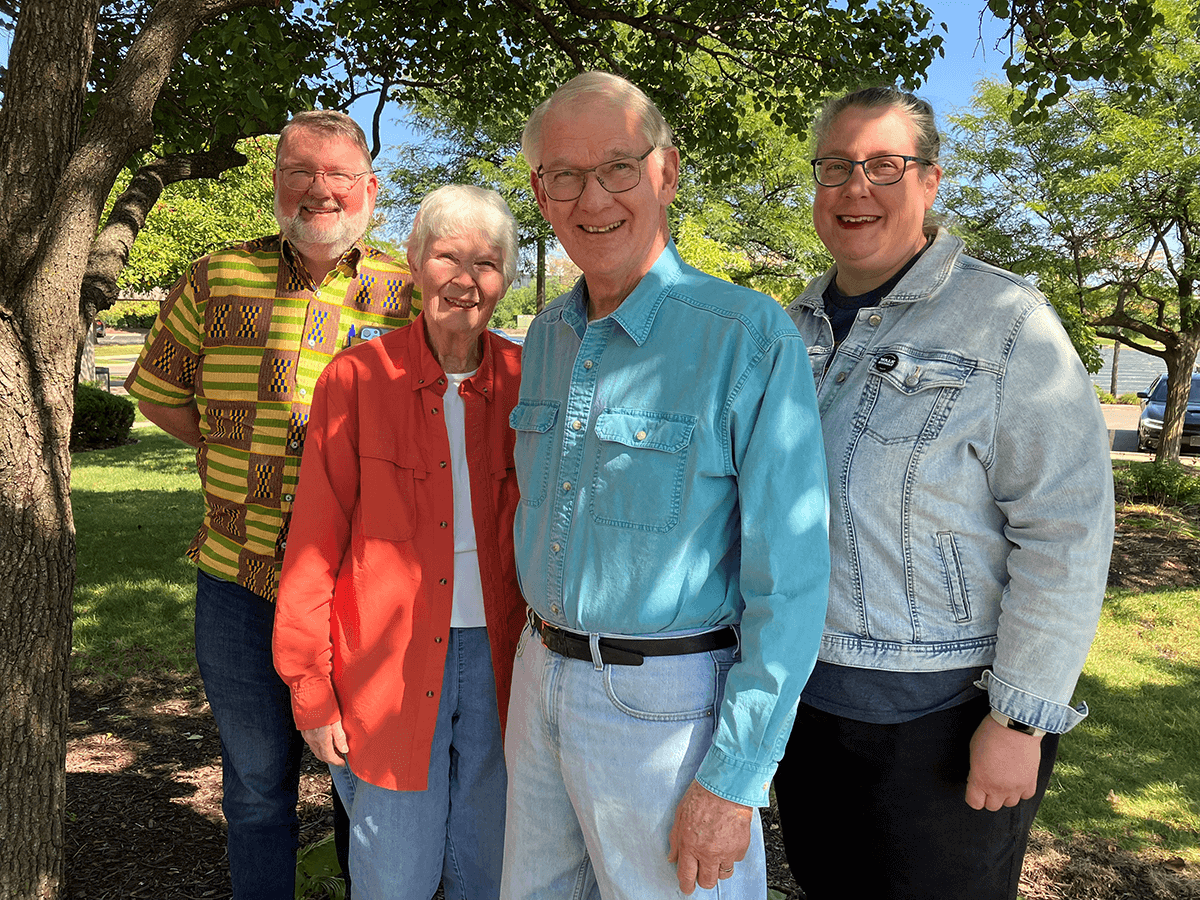
John and Candace with one of their sons and his wife
What is MONJUVI?
MONJUVI (tafasitamab-cxix) is a prescription medicine given with lenalidomide to treat adults with certain types of diffuse large B-cell lymphoma (DLBCL) that has come back (relapsed) or that did not respond to previous treatment (refractory) and who cannot receive a stem cell transplant.
It is not known if MONJUVI is safe and effective in children.
The approval of MONJUVI is based on a type of response rate. There is an ongoing study to confirm the clinical benefit of MONJUVI.
IMPORTANT SAFETY INFORMATION
What are the possible side effects of MONJUVI?
MONJUVI may cause serious side effects, including
- Infusion reactions. Your healthcare provider will monitor you for infusion reactions during your infusion of MONJUVI. Tell your healthcare provider right away if you get fever, chills, flushing, headache, or shortness of breath during an infusion of MONJUVI
- Low blood cell counts (platelets, red blood cells, and white blood cells). Low blood cell counts are common with MONJUVI, but can also be serious or severe. Your healthcare provider will monitor your blood counts during treatment with MONJUVI. Tell your healthcare provider right away if you get a fever of 100.4 °F (38 °C) or above, or any bruising or bleeding
- Infections. Serious infections, including infections that can cause death, have happened in people during treatment with MONJUVI and after the last dose. Tell your healthcare provider right away if you get a fever of 100.4 °F (38 °C) or above, or develop any signs or symptoms of an infection
The most common side effects of MONJUVI include
- Feeling tired or weak
- Diarrhea
- Fever
- Swelling of lower legs or hands
- Respiratory tract infection
- Decreased appetite
These are not all the possible side effects of MONJUVI. Your healthcare provider will give you medicines before each infusion to decrease your chance of infusion reactions. If you do not have any reactions, your healthcare provider may decide that you do not need these medicines with later infusions. Your healthcare provider may need to delay or completely stop treatment with MONJUVI if you have severe side effects.
Before you receive MONJUVI, tell your healthcare provider about all your medical conditions, including if you
- Have an active infection or have had one recently
- Are pregnant or plan to become pregnant. MONJUVI may harm your unborn baby. You should not become pregnant during treatment with MONJUVI. Do not receive treatment with MONJUVI in combination with lenalidomide if you are pregnant because lenalidomide can cause birth defects and death of your unborn baby
- You should use an effective method of birth control (contraception) during treatment and for at least 3 months after your last dose of MONJUVI
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with MONJUVI
- Are breastfeeding or plan to breastfeed. It is not known if MONJUVI passes into your breastmilk. Do not breastfeed during treatment and for at least 3 months after your last dose of MONJUVI
You should also read the lenalidomide Medication Guide for important information about pregnancy, contraception, and blood and sperm donation.
Tell your healthcare provider about all the medications you take, including prescription and over- the-counter medicines, vitamins, and herbal supplements.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to MORPHOSYS US INC. at (844) 667-1992.
Please see the full Prescribing Information, including Patient Information, for additional Important Safety Information.
If you, like John, have DLBCL that came back or didn’t respond to the first treatment (relapsed or refractory DLBCL), start a conversation with your healthcare team about your options. To learn more about Monjuvi, relapsed or refractory DLBCL and for support and resources, visit www.Monjuvi.com.
These participants were compensated for their time.
A note from Blood-Cancer.com: The content of this article was provided by our sponsor. Blood-Cancer.com does not specifically endorse or recommend the program, product, medications or therapies discussed in this article.
MONJUVI is a registered trademark of MorphoSys AG.
MorphoSys is a registered trademark of MorphoSys AG.
Incyte is a registered trademark of Incyte.
November 2023 RC-US-TAF-01890
